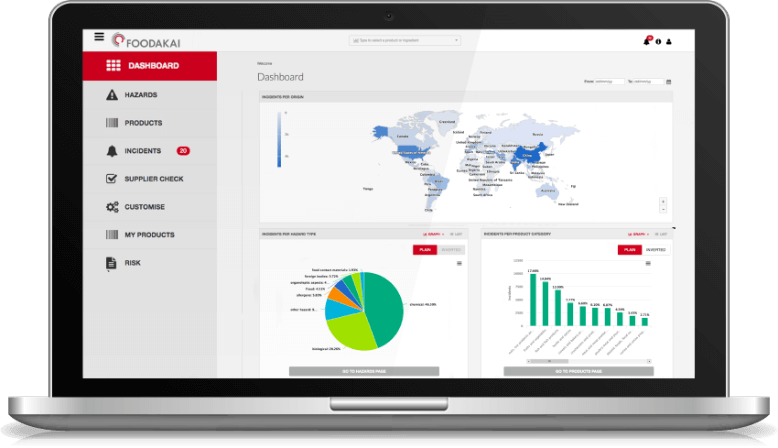
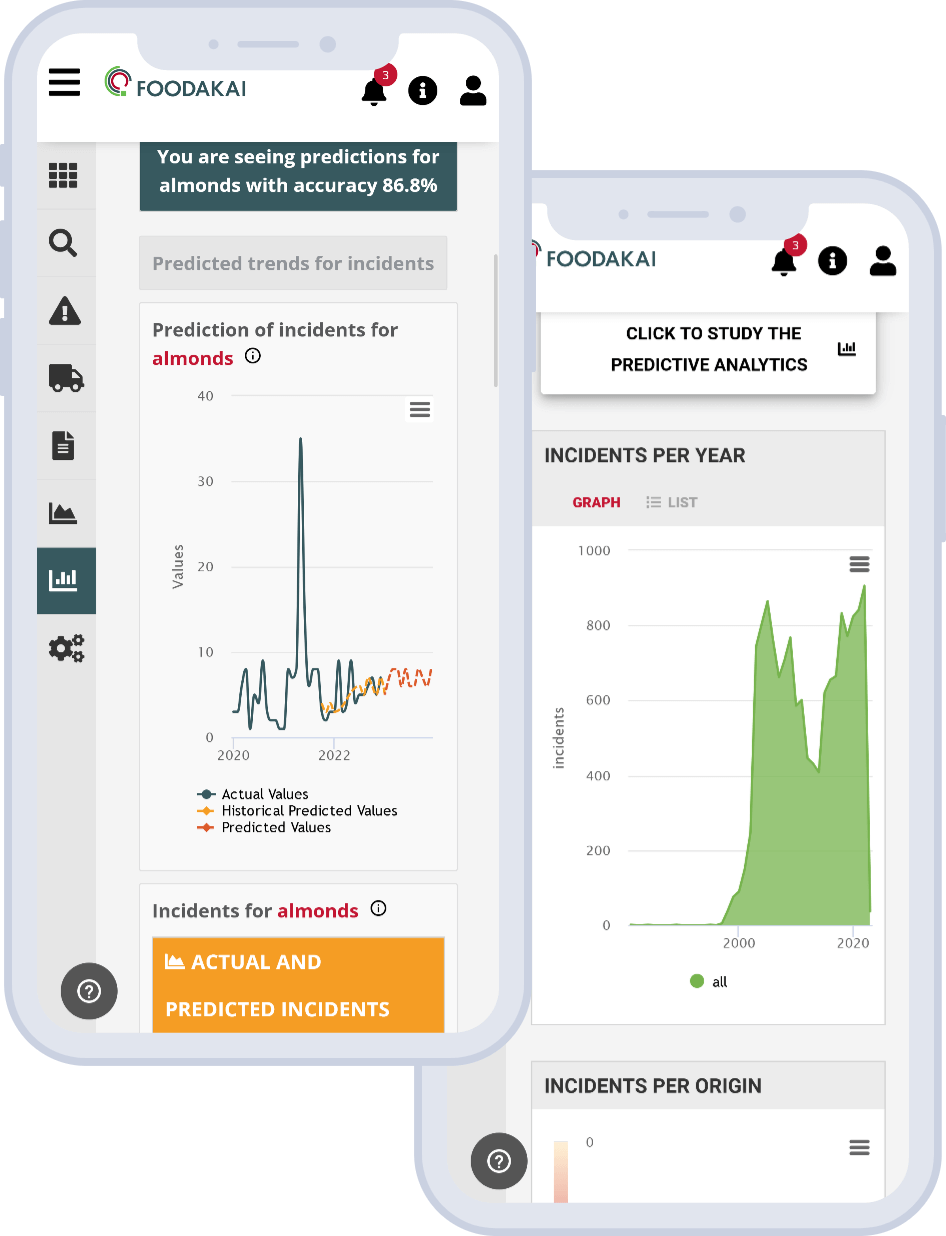
How Does Mercury End Up in Your Tuna Salad?

Whether you’re a fan of sushi, tuna salad, or shrimp pasta, there’s rarely any reason to cut down on seafood. Fish is considered a highly nutritious food, packed with protein and heart-healthy oils that offer protection against cardiovascular diseases, while also helping neurological development.
Nevertheless, it is highly likely that you may have come across warnings against consuming too much tuna, for example. While tuna is a great source of protein, omega-3 fatty acids, and B vitamins, it is generally considered to be riskier when it comes to heavy metal concentrations, particularly mercury.
Mercury can be found in trace amounts in almost all fish and shellfish, along with other contaminants called persistent organic pollutants (POPs). As larger fish consume smaller ones in the food chain, mercury and POP concentrations rise, leading to the highest levels being found in large, predatory deep-ocean fish. This is why consumers are often advised to avoid consuming large fish such as shark, swordfish, tilefish, and king mackerel due to their high mercury content. Although it’s not advisable to entirely cut out seafood from our diet, the US FDA advises consuming up to 12 ounces (equivalent to two average meals) of cooked seafood per week, encompassing a variety of fish types, while steering clear of such large predatory ocean fish. Some species with the lowest levels of mercury include bottom-feeding scallops, clams, and shrimp.
It is important to point out that most of the mercury that finds its way into the ocean from land-based or airborne sources is elemental mercury, which is relatively harmless as living organisms can quickly eliminate it. The form of mercury that accumulates to toxic levels in fish is known as monomethylmercury, or simply methylmercury. This type of mercury has a methyl group (CH3) attached to the mercury atom, which makes it extremely hazardous and bioaccumulative, meaning it tends to accumulate in the organism. Methylmercury is liked to one of the most notorious poisoning cases in recent history, namely Minamata Bay in Japan, as a chemical plant dumped tons of industrial wastewater into the bay. The so-called Minamata disease was first discovered in 1956 and resulted in animal and human deaths that continued for 36 years.
In this case, the source of the methylmercury was clear; however, if elemental mercury is not itself harmful, how does it end up causing such dangerous levels of methylmercury poisoning? Although some of the elemental mercury in the sea comes from natural causes like volcanic eruptions and forest fires, around two-thirds of it comes from human activities, mostly from the burning of fossil fuels, as well as gold mining. Burning coal releases 160 tons of mercury a year into the air in the US alone, which then finds its way into the oceans as rain. Of course, the heavy metal can be directly dumped into waterways by factories such as paper mills, battery manufacturers, or mining operations. It is estimated that between 80,000 and 450,000 metric tons of mercury enter the oceans every year, with around 66% of that ending up in shallower waters.
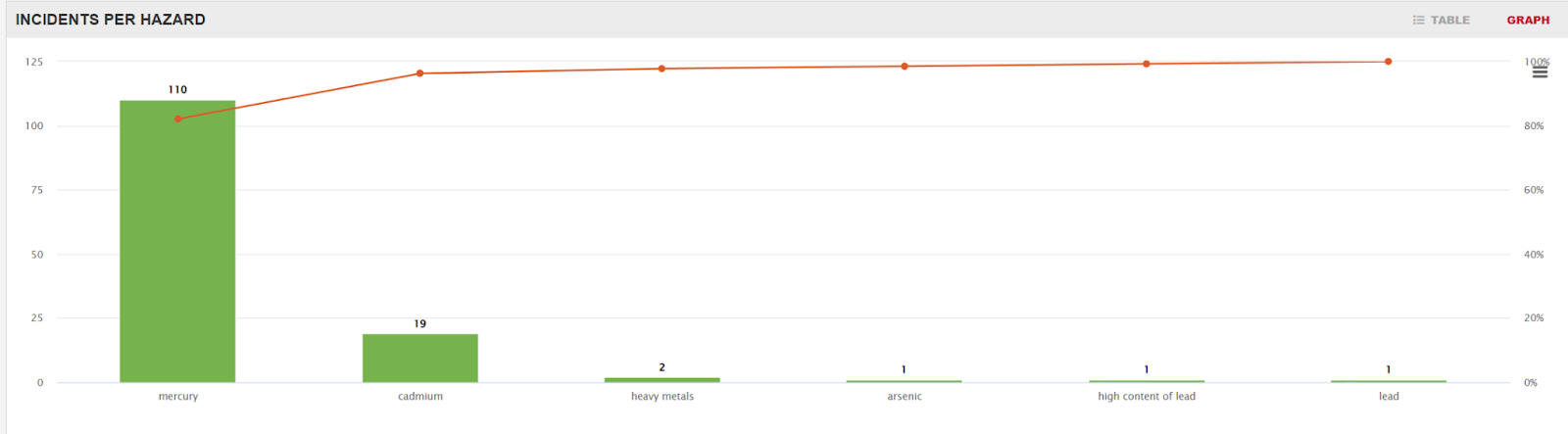
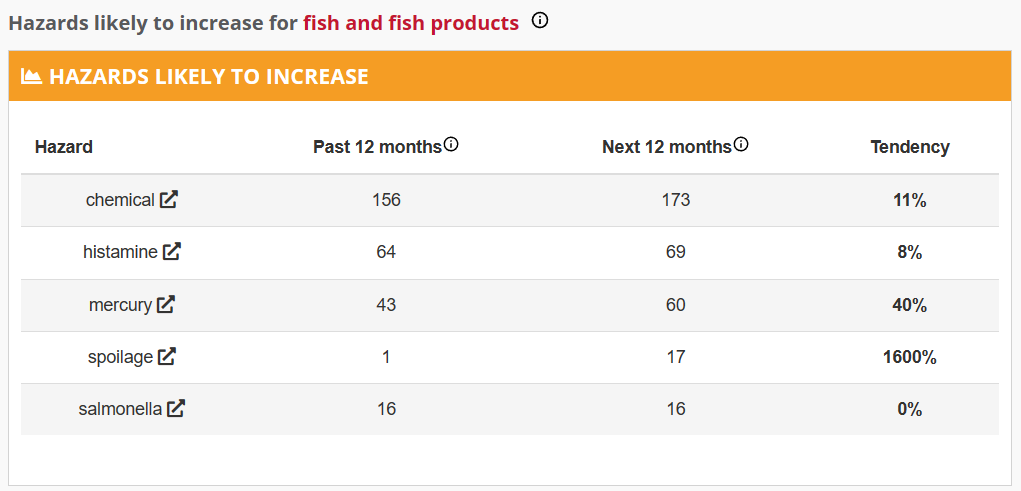
But even knowing this scale of mercury contamination is not enough to justify why large fish contain large amounts of methylmercury. Researchers believe that the conversion of mercury to methylmercury is biotic, meaning it is carried out by living things, one theory being that some species of bacteria produce the hazardous substance as a byproduct of their respiration. A few centimeters deep in the sediment, where oxygen is scarce, microbes rely on anaerobic respiration, often utilizing a chemical reaction called sulfate reduction. When the seawater contains mercury, the conditions are perfect for methylmercury production, as sulfide helps mercury enter cells. The substance then gets diffused from the sediments into open water, where it is absorbed by phytoplankton, initiating its journey up the food chain. Mercury adheres to fish fat cells, penetrating the lipid membranes of cells and dispersing throughout the nearby tissue. Due to its strong affinity for proteins and amino acids in muscles, as smaller fish are consumed by larger fish the mercury accumulates, multiplying by approximately tenfold at each step. Consequently, the longer a fish lives, the more mercury it accumulates.
Mercury poisoning, resulting from food consumption or air pollution, leads to the near-total absorption of mercury into the bloodstream, which then rapidly spreads throughout the body. The brain accumulates the highest concentration of mercury, making it a neurological toxin. Over time, mercury levels can build up in the human body, exacerbating its effects. Advanced mercury poisoning in adults can cause hearing and speech difficulties, lack of coordination, muscle weakness, nerve loss in hands and face, trouble walking, and vision changes. High mercury levels can result in long-term, sometimes permanent, neurological changes, posing significant risks to young children during development. Mercury exposure may cause developmental issues in the brain, affecting physical functions such as motor skills, and potentially leading to learning disabilities in children. In adults, mercury poisoning can lead to permanent brain and kidney damage, as well as circulatory failure.

Despite all this, we know that omega-3 fatty acids, known for their anti-inflammatory properties and potential in reducing the risk of chronic conditions such as arthritis and heart issues, cannot be produced by the human body and must be obtained through diet. Oily, coldwater fish like salmon, herring, mackerel, anchovies, and menhaden contain high amounts of omega-3. These fish acquire their omega-3 fatty acids primarily from marine algae and plankton. Farmed fish, lacking access to these omega-3-rich foods, receive omega-3 through added fish oil in their food. About 80% of fish oil produced is, in fact, consumed by the fish farming industry, ensuring farmed fish have similar omega-3 content as their wild counterparts. However, this practice is not foolproof, as wild fish oil may contain heavy metal pollutants like mercury, dioxin, and other toxic chemicals like polychlorinated biphenyls (PCBs). Short-term exposure to high dioxin levels can cause altered liver function and skin lesions, while long-term exposure may impair the reproductive, immune, endocrine, and developing nervous systems.
So what about that tuna salad? It looks like humans are not ready to stop dumping tons of mercury into the sea just yet; therefore, that tuna salad should be eaten in moderation, and in any case not every day.